Archived documents
____________________________________________________
DATE: 19.04.20. VERSION 3.0
As the current Covid-19 pandemic evolves, we are updating guidance with regard to investigation of suspected colorectal cancer. NHS England have issued guidance on the organisation of radiological services during the pandemic which includes setting up standard operating procedures for all classes of patients, including urgent outpatient groups (https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/03/specialty-guide-radiology-and-coronavirus-v1-20-march-2020.pdf).
Whilst many hospitals have seen a significant reduction in two-week referral activity, such deferral is creating a rapidly growing backlog.
Current guidance documents by the British Society of Gastroenterology (BSG), Joint Advisory Group on GI endoscopy (JAG), British Society of Gastrointestinal and Abdominal Radiology and the Association of Coloproctology of Great Britain and Ireland (ACPGBI), recommend careful triage of urgent lower GI cancer referrals to prioritise those requiring urgent investigation and recommend cessation of non-essential endoscopy (https://www.bsg.org.uk/covid-19-advice/endoscopy-activity-and-covid-19-bsg-and-jag-guidance/) ( https://www.acpgbi.org.uk/news/joint-acpgbi-bsg-and-bsgar-considerations-for-adapting-the-rapid-access-colorectal-cancer-pathway-during-covid-19-pandemic/).
Standard CT abdomen and pelvis has been prioritised over CT colonography (CTC), the rationale for which is detailed in our previous guidance (https://www.bsgar.org/society/covid-19-and-bsgar-updates-1/covid-19-and-bsgar-update-archive/).
Whilst the principles of the prior guidance still hold, it is clear careful planning is required to pre-empt an insurmountable backlog in suspected lower GI cancer referrals from building up.
The greater sensitivity of CT colonography than unprepared CT for colorectal cancer is well established (approximately 95% vs 75-80%), and under current guidance, deferred luminal investigation is recommended in the case of a negative standard CT in such patients.
Current guidance also states that a reduced CTC service may continue with local stakeholder agreement; even so, it is clear CTC activity has reduced considerably in the NHS.
The impact of the current pandemic on radiological capacity will differ between hospitals but it is apparent that CT capacity does exist in many (both scanner and staffing). This has been bolstered by commissioning of additional capacity from the private sector. In recent days, several sectors have started re-introducing a reduced CTC service for patients at high risk of colorectal cancer based on careful clinical triage, usually including FIT testing and symptom review.
Given the superiority of CTC over standard CT for detecting colorectal neoplasia and the need to address the backlog of referral activity, it is therefore recommended radiological services thoroughly review their current capacity, aiming to re-introduce a reduced CTC service if this is deemed practicable and safe. Such a review should include:
Please click here for a pdf of this document.
BSGAR Committee – 19th April 2020
Prof Stuart Taylor - President
Dr Raneem Albazaz - Secretary
Dr Chris Clarke - Treasurer
Dr Andrew Plumb - Research Officer
Dr James Stephenson - Audit Officer
Dr Jamie Franklin - Education Officer
Dr Cindy Chew - Standards Officer
Dr Emma Helbren - Liaison Officer
DATE: 25.03.20. VERSION 1.0
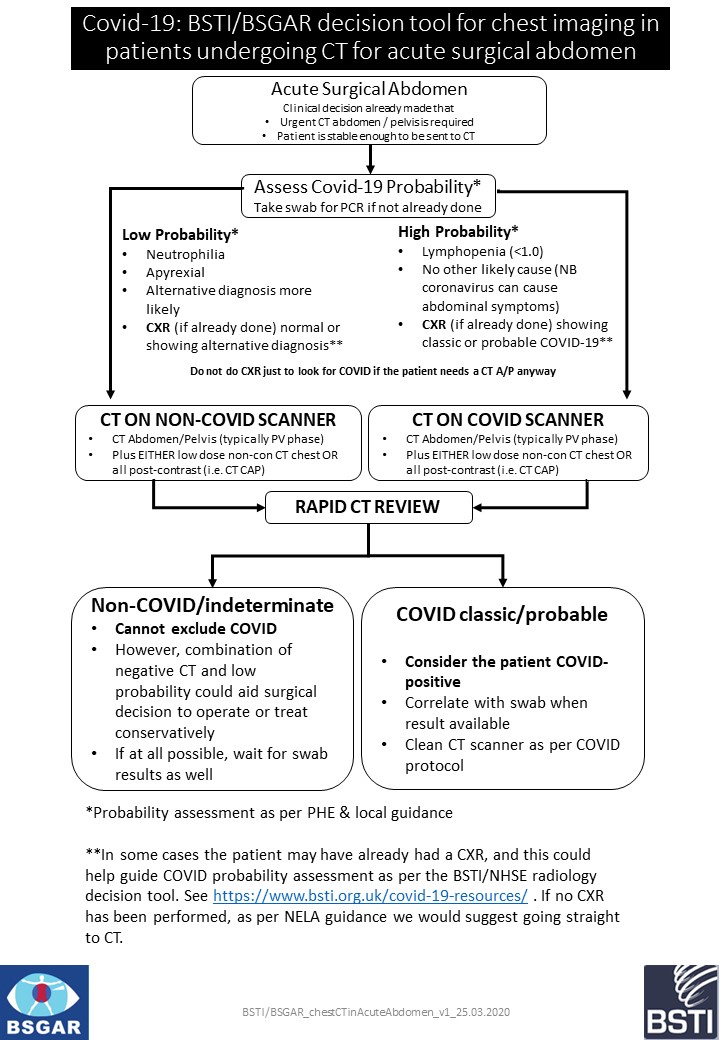
Rationale for the tool
• In the patient with an acute abdomen requiring potential emergency surgical intervention, intubation and ventilation could be aerosol generating.
• Reports are also emerging of increased mortality in Covid 19 positive patients in the setting of the acute surgical abdomen. As such, it may be useful to offer increased diagnostic confidence for Covid 19 in this setting, as it may influence the timing and approach to surgery.
• CT may help identify patients with Covid 19 before swab results are available; its sensitivity relative to RT PCR has been quoted as 97% in high risk patients with respiratory symptoms. Although this is almost certainly an overestimate, a CT suspicious for Covid 19 in the emergency acute abdomen setting could be taken to suggest Covid 19.
• CT is only 54% sensitive in asymptomatic patients who are RT PCR positive for SARS nCOV 2 (Inui et al, Radiology Cardiothoracic Imaging March 2020 https://doi.org/10.1148/ryct.2020200110 ); as such, a negative CT cannot be considered to have sufficient negative predictive value to exclude Covid 19 . However, in the emergency acute abdomen setting, a negative CT as well as low probability of Covid 19 could aid the confidence in the surgical decision to take the patient to theatre or manage the patient conservatively.
• As such, we advocate CT thorax (entire chest) opportunistically, if the clinical decision has already been made to send the patient for CT abdomen and pelvis, assuming cardiovascular and pulmonary stability.
• We stress that this recommendation does not apply to patients in whom abdominal CT (or MRI) is being performed for other reasons, or electively.
• We would recommend against extending the abdominal scan to only the caudal half of the thorax for two reasons: (1) an abnormality may be detected at the cranial most aspect of the chest acquisition, leading to uncertainty; (2) although it would be rare for Covid 19 pulmonary findings to be solely located in the cranial half of the thorax, this is reported.
• We also suggest that rapid review by the acute reporting radiologist (ideally on the scanner table, if feasible) is obtained, to help guide probability of Covid 19 with respect to cleaning the scanners and directing the patient’s subsequent disposition ( Covid vs non Covid bays). BSTI/BSGAR_chestCTinAcuteAbdomen_v1_25.03.2020
DATE: 25.03.20. VERSION 2.0
The British Society of Gastroenterology (BSG) and Joint Advisory Group on GI endoscopy (JAG) originally published guidance on the use of endoscopy during the current Covid-19 pandemic on March 17th and March 20th (https://www.bsg.org.uk/covid-19-advice/endoscopy-activity-and-covid-19-bsg-and-jag-guidance/) . The guidance is being kept under review, and has been updated on March 23rd.
In summary, BSG-JAG is currently recommending that ALL non-emergency endoscopy stops immediately.
In line with BSG-JAG, we recommend that CT colonography should also stop unless there is explicit local agreement amongst all relevant stakeholders that capacity exists to continue a reduced service.
This local decision must consider the safety of patients and wider public, as well as healthcare staff, while maintaining capacity and resource. The potential risk of transmission of Covid-19 in faeces, and the risk of the disease particularly in older patients is described in our statement 1.0 dated 21/03/20. However, now that endoscopy is likely to cease, not all patients requiring investigation will be the frail elderly group typically referred for CTC.
Our expectation is that CTC should cease in most hospitals, but acknowledge capacity may currently still exist in certain centres.
Patients requiring investigation for possible colorectal cancer under a 2 week wait referral pathway should be risk-stratified using symptoms and FIT testing (assuming such testing is available). One possible option would be:
- FIT <10 then discharge
- FIT 10-150 then reassess post-COVID (or, if service capacity allows, prompt CT abdo/pelvis and if negative reassess post-COVID with repeat FIT or a luminal test)
- FIT >150 then prompt CT abdo/pelvis, and if negative reassess post-COVID with repeat FIT or a luminal test
Precise investigation pathways will depend on local availability of FIT testing, demand, capacity and staffing, and should be discussed with appropriate medical professionals including primary care providers, radiologists, gastroenterologists, endoscopists and lower GI surgeons. Infectious diseases specialists will be able to provide crucial information regarding risks of transmission to staff and patients.
The risk to patients of contracting Covid-19 while attending for investigation must be considered; given the high mortality in patients >70 years, it may be appropriate to defer investigation in patients older than this, even if a FIT is >150.
Those services who maintain some capacity to perform CTC should decide how best to implement the test within any local pathway. Ultimately, each service (or region) must define their own pathways depending on their current situation, capacity and levels of service provision (particularly ongoing provision of cancer surgery). Pathways must be reviewed on a regular basis.
- CT colonography is used as an alternative to endoscopy, in older and/or frail patients, and those with co-morbidities such as cardiovascular and respiratory disease. The incidence of a colorectal cancer diagnosis in this population is low for those referred via a 2 week wait pathway (3-7%) and the incidence on CTC is in the lower part of this range. This compares to a current Covid-19 mortality rate of around 5% in those aged 70-79 and over 9% in those aged 80+. The risks of transmission of Covid-19 must therefore be considered in all clinical decision-making regarding CTC.
- Since JAG-BSG have advised non-emergency colonoscopy should stop, there is now a large cohort of otherwise healthy patients with colorectal symptoms who ordinarily would have had colonoscopy. This is a very different patient population to those typically referred for CTC.
- Covid-19 RNA is excreted in faeces at the time of infection and may persist for at least 2 weeks after respiratory samples become negative. Faeces should be considered as infectious for transmission of Covid-19. Currently lower GI endoscopy is not considered an aerosol prone procedure (AGP), although this remains under review. CTC should be considered in the same risk category as lower GI endoscopy, and equivalent levels of PPE should be implemented. This should include appropriate hand washing procedures, gloves, apron and surgical mask. Strong consideration should be made to the use of a protective visor/goggles, accepting the risk of splash events will likely be less than lower GI endoscopy. Although it may not be practicable, consideration should be given to designating a “clean scanner” for patients without known or suspected Covid-19.
- CTC needs to be performed by highly trained radiographers and reported by dedicated trained radiologists and radiographers; at this time of unprecedented demand on services this cannot be guaranteed. The time taken to scan the patient and report the subsequent images is also significantly more than that of a standard CT abdomen and pelvis adding further pressures.
- Standard CT abdominal and pelvis (with or without prolonged oral contrast) is an accepted alternative to CT colonography for detecting established colonic cancer, particularly in patients with suggestive symptoms or signs. The diagnostic accuracy of standard CT for tumours falls significantly below that of CT colonography.
- The risk of leaving an undiagnosed cancer in situ for an as yet undefined period (assumed to be between 3 and 6 months) is unknown. The short-term risk is largely mitigated by the use of prompt CT abdo/pelvis. The longer term risk (e.g. an early stage cancer becoming node-positive, or interim development of metastases) is presently unknown.
Please click here for a pdf of this document.
DATE: 21.03.20. VERSION 1.0
On March 17th 2020 (updated March 20th), the British Society of Gastroenterology (BSG) and Joint Advisory Group on GI endoscopy (JAG) published guidance on the use of endoscopy during the current Covid-19 pandemic (https://www.bsg.org.uk/covid-19-advice/endoscopy-activity-and-covid-19-bsg-and-jag-guidance/) . The guidance will be kept under review.
As part of the need to rationalise imaging activity, clarity is needed on the use of CT colonography (CTC) as an alternative to lower gastrointestinal endoscopy. The background on rationalising colonic investigation is provided by the joint BSG-JAG statement which should be read in conjunction with this document.
The aims are to protect patients and wider public, as well as healthcare staff, while maintaining capacity and resource.
- CT colonography is used as an alternative to endoscopy, in older and/or frail patients, and those with co-morbidities such as cardiovascular and respiratory disease. The incidence of a colorectal cancer diagnosis in this population is low for those referred via a 2 week wait pathway (3-7%) and the incidence on CTC is in the lower part of this range. This compares to a current Covid-19 mortality rate of around 5% in those aged 70-79 and over 9% in those aged 80+. The risks of transmission of Covid-19 must therefore be considered in all clinical decision-making regarding CTC.
- Covid-19 RNA is excreted in faeces at the time of infection and may persist for at least 2 weeks after respiratory samples become negative. Faeces should be considered as infectious for transmission of Covid-19. Currently lower GI endoscopy is not considered an aerosol prone procedure (AGP), although this remains under review. CTC should be considered in the same risk category as lower GI endoscopy, and equivalent levels of PPE should be implemented. This should include appropriate hand washing procedures, gloves, apron and surgical mask. Strong consideration should be made to the use of a protective visor/goggles, accepting the risk of splash events will likely be less than lower GI endoscopy. Although it may not be practicable, consideration should be given to designating a “clean scanner” for patients without known or suspected Covid-19.
- Standard CT abdominal and pelvis (with or without prolonged oral contrast) is an accepted alternative to CT colonography for detecting established colonic cancer, particularly in patients with suggestive symptoms or signs. The diagnostic accuracy of standard CT for tumours falls significantly below that of CT colonography.
- The English national bowel cancer screening program (BCSP) may well pause, and in some regions, there has already been temporary suspension.
- The 2-week cancer referral pathways will likely change, with increased use of FIT testing in primary care to triage patients.
• CT colonography should not be performed in known or suspected Covid-19 patients, nor in those recently recovered from the infection
Many hospitals have instigated a triaging system to prioritise medical imaging at the current time. For consistency we have used the categories proposed by the joint BSG JAG guidelines
• Needs to continue;
• Defer until further notice;
• Needs discussion (possibly case-by-case, at consultant level)
The list below is not exhaustive, nor prescriptive and is provided as a guide.
Needs to continue
• Imaging for potentially life-threatening complications of colorectal cancer such as acute obstruction or perforation. Standard CT abdomen and pelvis should be performed in such patients
Defer until further notice
• All referrals for non-specific abdominal symptoms which falls outside the 2 week wait referral process
• Polyp follow up (i.e. repeat CTC for polyps left in situ) and colorectal polyp surveillance (i.e. CTC performed in patients with a personal history of previous polypectomy)
• Suspected benign colonic disease such as non-acute diverticular disease / road mapping of diverticular disease
Needs discussion or agreed pathways
• Two week wait referrals
• Symptomatic patients with an elevated FIT result (precise threshold subject to local agreement)
It is strongly recommended that hospital services should create an investigation pathway for patients in this category following direct consultation between appropriate medical professionals including primary care providers, radiologists, gastroenterologists, endoscopists and lower GI surgeons. This pathway, with full instruction on PPE for included investigations, must be disseminated to all appropriate staff including booking and reception teams, radiographers, portering staff and health care assistants.
As noted above, the 2-week cancer referral pathways will likely change with increased use of FIT testing in primary care to triage patients.
Depending on local circumstances it may be appropriate to replace some or all CT colonography referrals with standard CT abdomen and pelvis, targeting a symptomatic colonic mass (for example predisposing to bowel obstruction), accepting the lower diagnostic accuracy. Such an approach will require careful consideration of the future management and investigation of such patients if the standard CT is normal, given the risks of undiagnosed colonic neoplasia. It is likely higher-risk patients (based on symptoms or level of FIT positivity) will need to undergo formal luminal testing in the future with either CT Colonography or lower GI endoscopy.
Many services have instigated triage of patients based on levels of symptoms and FIT; for example, performing CT colonography in these with FIT levels 10-150 and lower GI endoscopy in those with levels >150. This may be further nuanced by patient age given the risk of morbidity and mortality of Covid-19 in the older population; although with altered investigation pathways, the demographics of patients referred for CTC may also shift towards a younger cohort. The most appropriate levels of FIT positivity that should trigger colonic investigation in the current situation are not yet defined.
Ultimately, each service (or region) must define their own pathways depending on their current situation, capacity and levels of service provision (ongoing provision of cancer surgery). Pathways must be reviewed on a regular basis.
Please click here for a pdf of this document.
Prof Stuart Taylor - President
Dr Raneem Albazaz - Secretary
Dr Chris Clarke - Treasurer
Dr Andrew Plumb - Research Officer
Dr James Stephenson - Audit Officer
Dr Jamie Franklin - Education Officer
Dr Cindy Chew - Standards Officer
Dr Emma Helbren - Liaison Officer